Vatengesi Vanonyanya Kutengesa Zvinopisa Na2so4 99% Sodium Sulphate Anhydrous
Tiri kutarisawo pakuvandudza hurongwa hwehutungamiriri hwezvinhu uye hweQC kuitira kuti tive nechokwadi chekuti tinokwanisa kuramba tichiwana purofiti yakakura kubva kukambani ine makwikwi makuru yeTop Suppliers Hot Selling Na2so4 99% Sodium Sulphate Anhydrous, Tinogamuchira vatengi vatsva nevakare vanobva kumativi ese ehupenyu kuti vatifonere kuti tiwane hukama hwemangwana hwebhizinesi uye kubudirira kwedu pamwe chete!
Tiri kutarisawo pakuvandudza hurongwa hwehutungamiriri hwezvinhu uye hweQC kuitira kuti tive nechokwadi chekuti tinokwanisa kuramba tichiwana purofiti yakakura kubva kukambani ine makwikwi akasimba. Kambani yedu inosimbirira papfungwa yekuti "Kutanga Kwemhando Yepamusoro, Kubudirira Kunogara Kwakagadzikana", uye inotora "Bhizinesi Rakavimbika, Mabhenefiti Ekubatana" sechinangwa chedu chinogoneka kugadzirwa. Nhengo dzese dzinotenda nemwoyo wese rutsigiro rwevatengi vese vekare nevatsva. Ticharamba tichishanda nesimba uye tichikupai zvinhu zvemhando yepamusoro nebasa repamusoro.



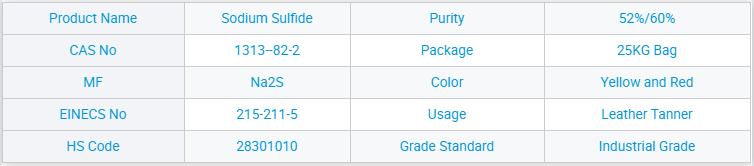

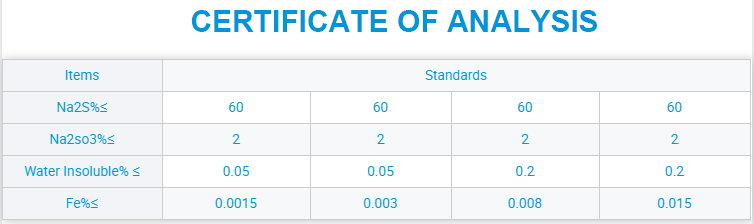



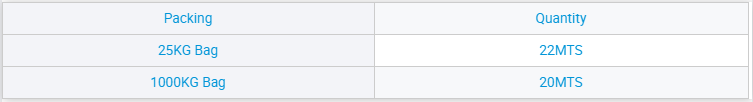



 Maitiro Ekugadzira Sodium Sulfide (Na₂S) Sodium Sulphate
Maitiro Ekugadzira Sodium Sulfide (Na₂S) Sodium Sulphate
Na₂SO₄ inoiswa muhopper kuburikidza neautomatic feeder yozoendeswa mukamuri rinopisa, uko Sodium Sulphate inofuridzwa uye inopiswa uchishandisa gasi remarasha. Kana tembiricha yasvika 884°C, sodium sulfate inonyunguduka kuita mvura. Na₂SO₄ yakanyungudutswa inova isina kugadzikana nemakemikari, uye maion eSO₄²⁻ anoora nyore nyore. Kuchinjana kwemakemikari kunoitika uchishandisa carbon monoxide kubva mugasi remarasha rine phosphorus, seizvi:
Na₂SO₄ + 4CO → Na₂S + 4CO₂
Kana murazvo uri muchoto ukachinja kubva pabhuruu kuenda patsvuku, shanduko yeCO inopedzwa. Pamusoro peiyi poindi, CO inoshanda chete semafuta ekuwedzera tembiricha. Kana tembiricha yasvika 1100°C, huwandu hushoma hweanthracite hunowedzerwa. Mugumo wemhinduro unoratidzwa nekuonekwa kwemurazvo weyero unopenya mukati mechoto. Maitiro emakemikari ndeekuti:
Na₂SO₄ + 2C → Na₂S + 2CO₂
Sodium Sulfate.




















