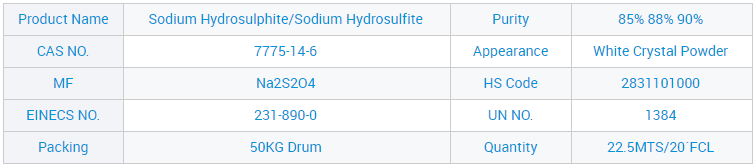
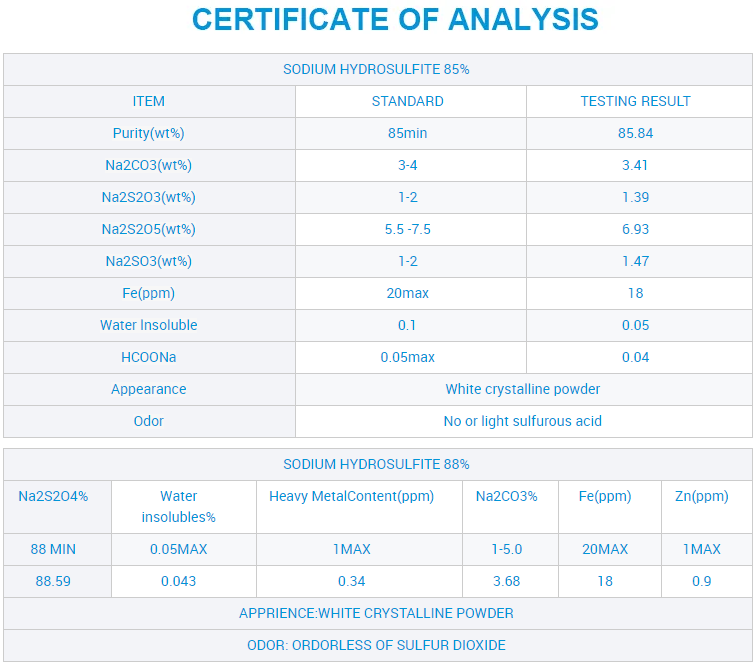
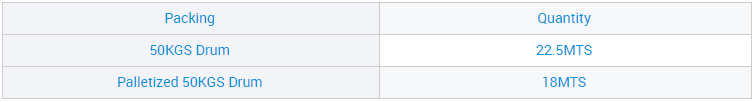
CAS 7775-14-6 Mutengo weSodium Hydrosulfite weKubatsira
Zvigadzirwa zvedu zvinozivikanwa zvakanyanya uye zvinovimbika nevatengi uye zvinogutsa zvishuwo zvehupfumi nemagariro evanhu zveCAS 7775-14-6 Mutengo weSodium Hydrosulfite weBeneficiation, "Kugadzira Zvigadzirwa zveUnhu Hwakanaka" ndicho chinangwa chekambani yedu chisingaperi. Tinoita zvese zvatinogona kuti tizadzise chinangwa chekuti "Ticharamba Tichifambirana Nenguva".
Zvigadzirwa zvedu zvinozivikanwa zvakanyanya uye zvakavimbika nevatengi uye zvinogutsa zvishuwo zvehupfumi nemagariro evanhu zvinogara zvichikura, Kuti mugone kushandisa ruzivo rwuri kuwedzera mukutengeserana kwenyika dzese, tinogamuchira vatengi vanobva kwese kwese online nekunze kwenyika. Pasinei nemhinduro dzakanaka dzatinounza, sevhisi yekukurukurirana inoshanda uye inogutsa inopihwa nechikwata chedu chehunyanzvi mushure mekutengesa. Rondedzero yezvigadzirwa uye ma parameter akazara uye chero rumwe ruzivo ruchatumirwa kwamuri panguva yekubvunza kwenyu. Saka iva nechokwadi chekuti matibata nesu nekutumira maemail kana kutifonera kana muine mibvunzo nezvekambani yedu. Munogonawo kuwana ruzivo rwekero yedu kubva pawebhusaiti yedu uye kuuya kukambani yedu kuti muwane ongororo yezvigadzirwa zvedu. Tave nechivimbo chekuti tiri kuda kugovana kubudirira kwedu uye kugadzira hukama hwakasimba nevashandi vedu mumusika uyu. Tiri kutsvaga mibvunzo yenyu.








Mibvunzo Inowanzo bvunzwa
Unoda rubatsiro here? Iva nechokwadi chekushanyira maforamu edu ekutsigira kuti uwane mhinduro dzemibvunzo yako!
Tinogona here kudhinda logo yedu pachigadzirwa?
Ehe, tinogona kuzviita. Ingotumirai dhizaini yelogo yenyu.
Munogamuchira maodha madiki here?
Ehe. Kana uri mutengesi mudiki kana kuti uri kutanga bhizinesi, tinoda kukura newe. Uye tinotarisira kushanda newe kwenguva refu.
Ko mutengo wacho wakaita sei? Unogona here kuita kuti ive yakachipa?
Tinogara tichitora rubatsiro rwemutengi sechinhu chinonyanya kukosha. Mutengo unogona kutaurwa nezvawo mumamiriro ezvinhu akasiyana, tiri kukuvimbisa kuti uchawana mutengo unokwikwidzana zvakanyanya.
Munopa mienzaniso yemahara here?
Zvinofadza kuti munogona kutinyorera wongororo dzakanaka kana muchida zvigadzirwa zvedu nebasa redu, tichakupai mimwe mienzaniso yemahara pakutenga kwenyu kunotevera.
Unokwanisa here kuendesa zvinhu nenguva?
Ehe, takaita basa iri kwemakore akawanda, vatengi vazhinji vanoita chibvumirano neni nekuti tinokwanisa kuendesa zvinhu nenguva uye kuchengetedza zvinhu zvakanaka!
Ndingashanyira kambani yenyu nefekitori kuChina here?
Ehe. Munogamuchirwa kushanyira kambani yedu muZibo, China. (Nzira yekutyaira ye1.5H kubva kuJinan)
Ndingaisa sei odha?
Munogona kungotumira mibvunzo kune chero mumiriri wedu wekutengesa kuti muwane ruzivo rwakadzama rweodha, uye isu tichatsanangura maitiro acho. Sodium Hydrosulfite Chemical Properties
a. Simba Rinodzikisa: Rongalite ine simba guru rekudzikisa uye inowanzo sanganiswa kuita sodium sulfite uye sodium sulfate.
b. Kusagadzikana uye Kuora: Rongalite haina kugadzikana uye inoora pasi pemamwe mamiriro ezvinhu:
Kukurumidza kuoxidation mumhepo: 2Na₂S₂O₄ + O₂ → 2Na₂SO₄ + 2SO₂↑
Maitiro eSodium Hydrosulfite mumushonga wemvura: 2Na₂S₂O₄ + H₂O → Na₂S₂O₃ + 2NaHSO₃
Kuora kwechinhu mumhepo ine hunyoro: 2Na₂S₂O₄ + O₂ + 2H₂O → 4NaHSO₄
Kuora nekukurumidza patembiricha >90°C: 2Na₂S₂O₄ → Na₂SO₃ + Na₂S₂O₅
Kubatidza uye kupisa kunongoerekana kwaitika patembiricha iri pamusoro pe250°C.
c. Kuora kweSodium Hydrosulfite muAcidic/Basic Media:
Inoora nekukurumidza mu acidic media: Na₂S₂O₄ + H₂SO₄ → Na₂SO₄ + SO₂↑ + S + H₂O
Yakasimba zvishoma mu alkaline media, asi inoora mu alkali yakasimba: 3Na₂S₂O₄ + 6NaOH → 5Na₂SO₃ + Na₂S + 3H₂O













